The Digital Fortress: A Pharma Engineer’s Comprehensive Guide to Mastering 21 CFR Part 11 Compliance in Automation

The Audit Anxiety in the “Pharmacy of the World”
If you drive through the industrial corridors of Baddi, Nalagarh, or Paonta Sahib—the beating heart of India’s pharmaceutical manufacturing—you will see world-class facilities churning out generics for the global market. Yet, inside the conference rooms of these massive plants, one acronym generates more anxiety than any production target or supply chain delay: USFDA.
For Indian pharmaceutical exporters, the United States Food and Drug Administration (USFDA) audits are the ultimate litmus test. In recent years, the focus of these audits has shifted aggressively from physical hygiene to Data Integrity.
Gone are the days when a wet signature on a paper batch record was enough. Today, your PLC (Programmable Logic Controller) and SCADA (Supervisory Control and Data Acquisition) systems are the primary witnesses to your process quality. If an auditor asks, “Who changed this sterilization setpoint at 3:00 AM?” and your HMI cannot provide a definitive, tamper-proof answer, you are staring down the barrel of a Form 483 observation or, worse, a Warning Letter.
This is where 21 CFR Part 11 comes in.
To the uninitiated, it reads like dry legal text. To the experienced Automation Engineer, it is the blueprint for building a credible, export-ready facility.
At Advance Engineers, we have spent years working with pharma majors in the Chandigarh and Himachal region, helping them bridge the gap between engineering reality and regulatory requirements. We know that compliance isn’t just about buying “Part 11 compliant software”; it’s about how that software is engineered, configured, and validated.
This comprehensive guide is designed for the Plant Head, the QA Manager, and the Automation Engineer. We will strip away the legalese and explore the practical, nuts-and-bolts implementation of 21 CFR Part 11 in your industrial automation systems.
Part 1: De-mystifying the Regulation
Title 21 CFR Part 11 is the FDA’s regulation regarding Electronic Records and Electronic Signatures (ERES).
In simple terms, it states that electronic records (data stored in your SCADA/Historian) and electronic signatures (approvals done via login) are considered just as legally binding and valid as paper records and handwritten signatures—provided specific conditions are met.
The regulation is divided into two main subparts relevant to us:
Subpart B – Electronic Records: How you create, maintain, and archive data securely.
Subpart C – Electronic Signatures: How you ensure that a specific action is irrefutably linked to a specific human being.
Why is this hard? Because standard industrial automation was originally designed for efficiency, not security. A standard HMI lets anyone walk up, press “Start,” and walk away. A standard CSV file export lets anyone open it in Excel, change a value from 80°C to 121°C, save it, and no one would ever know. Part 11 forces us to lock these doors.
Part 2: The Core Pillar—Data Integrity and ALCOA+
Before diving into the PLC logic, we must understand the philosophy behind the rule: ALCOA+. This is the framework auditors use to judge your system. If your automation solution doesn’t satisfy these principles, it is not compliant.

A – Attributable: Every piece of data must be traced back to the person or system that created it. (No generic “Operator” logins).
L – Legible: The data must be readable and permanent throughout its lifecycle.
C – Contemporaneous: Data must be recorded at the time the event occurred. (No back-dating logs).
O – Original: The first capture of data is the source of truth.
A – Accurate: The data must be error-free and unaltered.
+ (Plus): Complete, Consistent, Enduring, and Available.
At Advance Engineers, when we design a SCADA architecture for a Sterile Injectable line or an OSD (Oral Solid Dosage) plant, we essentially build a “Digital Chain of Custody” that satisfies ALCOA+ at every step.
Part 3: Engineering Compliance – The Technical Implementation
This section details how we translate these regulations into actual engineering features within platforms like Siemens WinCC, Rockwell FactoryTalk View SE, or Wonderware System Platform.
1. STRICT Access Control & User Management
The days of a shared HMI password written on a sticky note are over.
Individual Accounts: Every operator, supervisor, and maintenance engineer must have a unique User ID.
Role-Based Access Control (RBAC): We configure security groups.
Operators can View process, Acknowledge alarms, and Start batches.
Supervisors can Change Setpoints and Modify Recipes.
Maintenance can Access PID tuning parameters.
Administrators can Manage users (but NOT run the process—segregation of duties).
Password Aging & Complexity: The SCADA system must force password changes every 30-90 days. It must reject simple passwords and lock the account after 3 failed attempts.
Auto-Logout: The system must automatically log out an inactive user after a set time (e.g., 10 minutes) to prevent unauthorized access if an operator walks away.
2. The “Black Box”: Audit Trails
This is the single most critical feature for an auditor. An Audit Trail is a secure, immutable chronological record of who did what, when, and why.
A compliant Audit Trail in a SCADA system must capture:
Timestamp: Date and Time (synced to a secure NTP server).
User ID: Who made the change?
Action: What happened? (e.g., “Setpoint Change”).
Variable Name: Which tag was affected? (e.g.,
Autoclave_Temp_SP).Old Value: What was it before? (e.g., “121.0”).
New Value: What is it now? (e.g., “121.5”).
Reason for Change: This is crucial. The system must force the user to select a reason from a pre-defined list (e.g., “Process Deviation,” “Calibration,” “Batch Change”) or type a manual comment before the value is accepted.
The Advance Engineers Standard: We configure Audit Trails to be “Read-Only” for everyone. Even the Administrator should not be able to delete or edit the audit logs. They are stored in encrypted SQL databases or tamper-evident proprietary file formats.
3. Electronic Signatures (The “Double Handshake”)
For critical actions—like starting a batch, approving a recipe, or acknowledging a critical alarm—a simple click is not enough. The system must demand an Electronic Signature.
This typically involves a pop-up window requiring two distinct identification components:
The User ID (Public).
The Password (Private).
This action essentially says, “I, John Doe, certify that I am authorizing this action at this time.” In our systems, this signature is permanently linked to the record of that batch.
4. Recipe Management and Version Control
Inconsistent batches are a quality nightmare. In a Part 11 compliant system, “Recipes” (the set of parameters defining a product) are locked down tight.
Version Control: If a recipe is modified, the system creates a new version (e.g., Version 1.0 -> 1.1).
Approval Workflow: A recipe created by a Junior Engineer cannot be used in production until it is electronically signed and “Approved” by a QA Manager.
Verification: When a batch starts, the PLC verifies that the loaded recipe matches the checksum of the approved recipe in the database, ensuring no parameters were tweaked in the background.
Part 4: The Danger of “Open Systems” and Data Storage
A common pitfall we see in older plants is the reliance on “Flat Files” like CSV or TXT files for data logging.
The Scenario: A SCADA system logs temperature data to a CSV file on the C: drive. At the end of the shift, the supervisor copies it to a USB stick.
The Compliance Violation: A user could open that CSV file, change a few temperature readings that were out of spec, save the file, and then present it to QA. There is no trace of the alteration. This is a critical data integrity failure.
The Solution: We implement Database-Centric Architectures.
SQL Server with Security: Data is logged directly into an SQL database. The database is password protected, and permissions are set so that only the SCADA Service Account can Write data. Human users have Read-Only access.
Encrypted Historians: We use specialized Historian software (like OSIsoft PI, FactoryTalk Historian, or Wonderware Historian) that compresses and encrypts data. It is mathematically impossible to modify a historical value without breaking the file’s integrity signature.
Part 5: Computer System Validation (CSV) and GAMP 5
Buying compliant software is only 50% of the battle. The other 50% is proving that it works. This is called Computer System Validation (CSV).
The pharmaceutical industry follows the GAMP 5 (Good Automated Manufacturing Practice) guide using the V-Model.
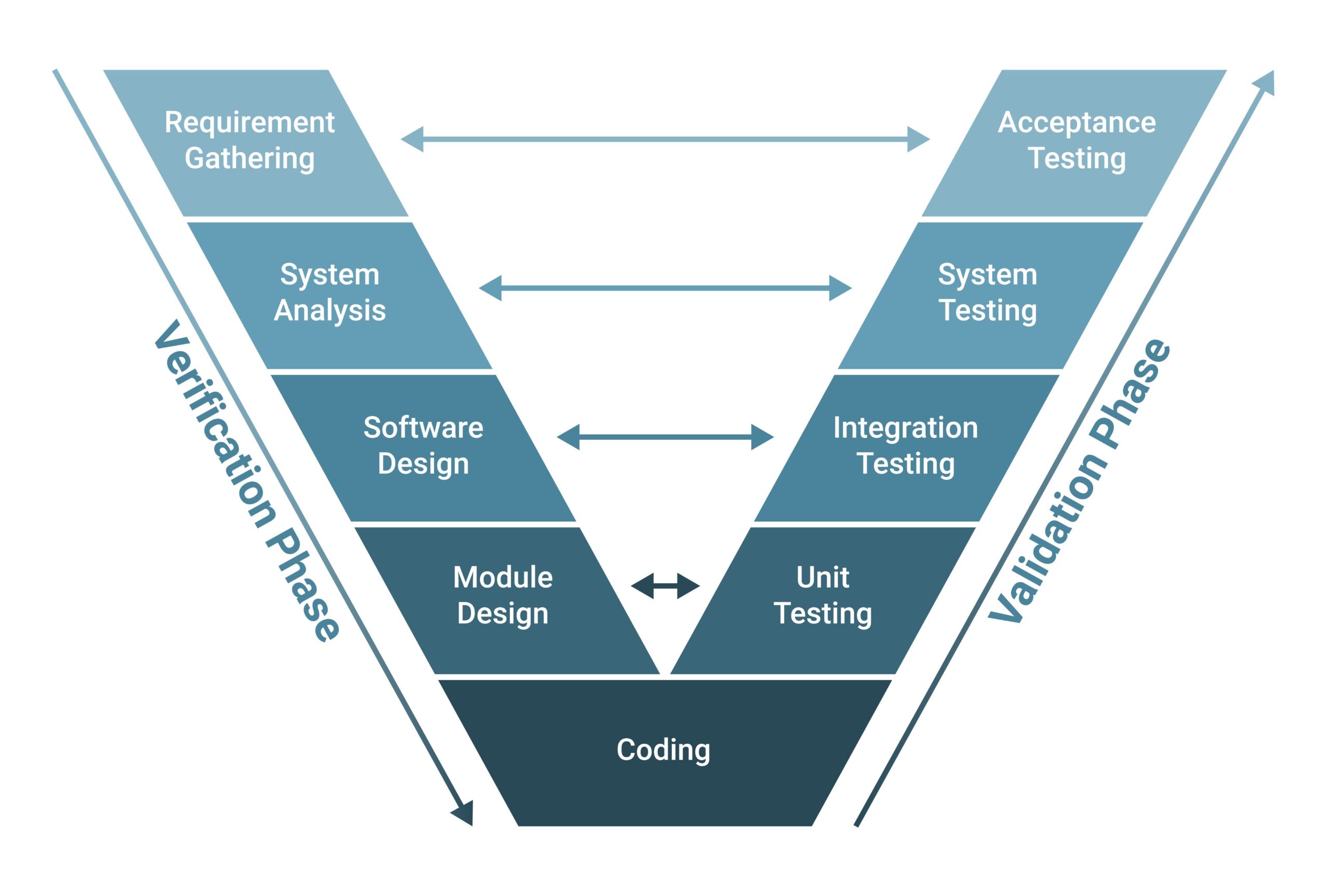
At Advance Engineers, we don’t just hand over the code; we provide the full documentation stack required for your validation master plan:
URS (User Requirement Specification): Helping you define exactly what the system must do.
FS/DS (Functional & Design Specifications): Documenting how our code meets your URS.
IQ (Installation Qualification): Verifying the hardware is installed correctly and the software is the correct version.
OQ (Operational Qualification): Testing every alarm, interlock, and security feature. (e.g., We deliberately try to log in with a wrong password to prove the system locks us out).
PQ (Performance Qualification): Verifying the system works under real production load.
Traceability Matrix: A document linking every Requirement -> Design Element -> Test Case.
Without this paperwork, your sophisticated SCADA system is just a “black box” to an auditor.
Part 6: Retrofitting Legacy Systems for Compliance
Many plants in India are running older machines that work perfectly mechanically but lack digital compliance.
Do you need to throw away the machine? No.
We specialize in “Compliance Retrofits.” We can install a “SCADA Overlay” or a “Data Integrity Gateway.”
We leave the existing PLC logic for machine control largely untouched (to minimize re-validation of the process).
We add a new, modern HMI/SCADA layer on top that handles User Management, Audit Trails, and Reporting.
We disable the local operator controls on the old panel and route all critical inputs through the compliant HMI.
This approach saves you the cost of a new machine while bringing you up to 21 CFR Part 11 standards.
Part 7: The Advance Engineers Advantage
Why trust Advance Engineers with your compliance?
Local Presence, Global Standards: Based in Chandigarh, we are minutes away from the major pharma hubs of Punjab and Himachal. We understand the local operational challenges but engineer to US/EU standards.
Multi-Platform Expertise: Whether your plant runs on Siemens, Rockwell, Mitsubishi, or Schneider, we have the in-house drivers and expertise to unify them into a compliant reporting structure.
IT/OT Convergence: We don’t just know PLCs; we know Databases, Networking, and Server Security. We bridge the gap between your shop floor and your IT department.
Conclusion: Compliance is a Culture, Not Just Code
21 CFR Part 11 is often viewed as a burden. However, when implemented correctly, it is a tool for excellence.
A compliant system doesn’t just satisfy an auditor; it gives you confidence.
Confidence that your batch records are accurate.
Confidence that your recipes are followed exactly.
Confidence that if a failure occurs, you can trace the root cause instantly.
In the high-stakes world of pharmaceuticals, “Data Integrity” is synonymous with “Product Safety.” There is no room for ambiguity.
Don’t let your next audit be a source of fear. Turn your automation data into your strongest asset.
Call to Action
Is Your Facility Audit-Ready?
Don’t wait for a Form 483 to reveal gaps in your data integrity.
At Advance Engineers, we offer a comprehensive Data Integrity Audit. Our experts will review your existing automation systems, identify compliance risks, and propose a practical roadmap to full 21 CFR Part 11 compliance.
Let’s build a system that auditors trust.